
Tackling slow set up times for paediatric clinical trials
Challenge
Clinical trials set up through the NHS is perceived to be slow, creating a barrier to initiating research, particularly with Industry sponsors. How do we address this issue to ensure we remain clinically competitive in the global clinical research landscape.
Piloting standard of care guidance to support trial costing
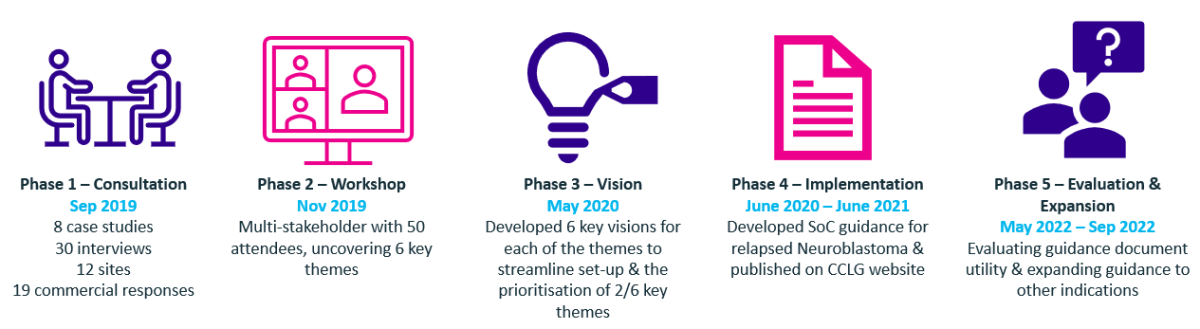
The NCRI Neuroblastoma sub-group, chaired by Dr Juliet Gray (Southampton ECMC) and includes other ECMC Paediatric Leads as members, worked together to develop a consensus standard of care guidance (SOC) document for early phase studies involving children and young people with relapsed neuroblastoma. This was designed to provide a clear definition of minimum standard of care requirements, where all other interventions falling outside of this, can then be classed as excess research costs, mitigating undue negotiations and subsequent delays to trial set-up.
The guidance can be accessed by CCLG members on their website.
Assessing the utility of the guidance
To evaluate the utility of this guidance document in supporting trial costing and to understand whether creating similar guidance for other paediatric cancer indications, could be beneficial, in partnership with the University of Birmingham Cancer Research Clinical Trials Unit, a survey was distributed to sites currently in set-up for the Ph III High Risk Neuroblastoma 2 trial (HR-NBL2/SIOPEN).
- 90% (9/10) indicated that they would use this guidance to support trial costing and set-up in future.
- All respondents would be in favour of similar documents being developed for other paediatric indications
- All respondents indicated that they would be in favour of the guidance being used to support with the costing of commercial/industry-sponsored trials.
Looking ahead - what next?
Following the outcomes of this survey, we will:
- Make the document widely available to study set-up teams
- Develop minimum SoC guidance documents for other paediatric cancer indications, starting off with those in CNS
- Work with partners such as the NIHR and HRA to understand how to reach a national consensus on the use of similar guidance, to support with more accurate costing of paediatric clinical trials
- Continue to assess the utility of the guidance, ensuring that it is fit-for-purpose
Send us your feedback
We want to ensure that this guidance is as useful as possible. The guidance document can be downloaded here.
Tell us your thoughts on the guidance, how it can suppot the costing of paediatric trials and any improvements that could be made.
You can share your feedback via this form, or please reach out directly via email. Please also share the guidance document with your colleagues involved in trial set-up who might also be able to provide valuable feedback.
