
New regulatory routes for cancer treatment in Britain
Speed, robust quality, safety and efficacy standards are an important feature in drug development and patient access. The departure of the UK from the EU in 2020 resulted in the Medicines and Healthcare products Regulatory Agency (MHRA) becoming a standalone regulator. This independence provided an opportunity to review the drug development and access pathway.
The ECMC PO convened a multi-stakeholder working group to bring together in one place the new regulatory approvals processes and the organisations involved.
Working group members
|
Sarah Blagden |
University of Oxford |
|
Paul Catchpole |
The Association of the British Pharmaceutical Industry |
|
Nicola Allen-Delingpole |
The Association of the British Pharmaceutical Industry |
|
Wendy Fisher |
Wendy Fisher Consulting |
|
Alison Hansford |
The Association of the British Pharmaceutical Industry |
|
Debbie Keatley |
Independent Cancer Patient Voices & National Cancer Research Institute |
|
Daniel O’Connor |
Medicines and Healthcare products Regulatory Agency |
|
Matthew Robinson |
Accelerated Access Collaborative |
|
Sharan Sandhu |
ECMC |
|
Kirsty Wydenbach |
Medicines and Healthcare products Regulatory Agency |
The end-to-end process of oncology drug development, approval and access in Great Britain.
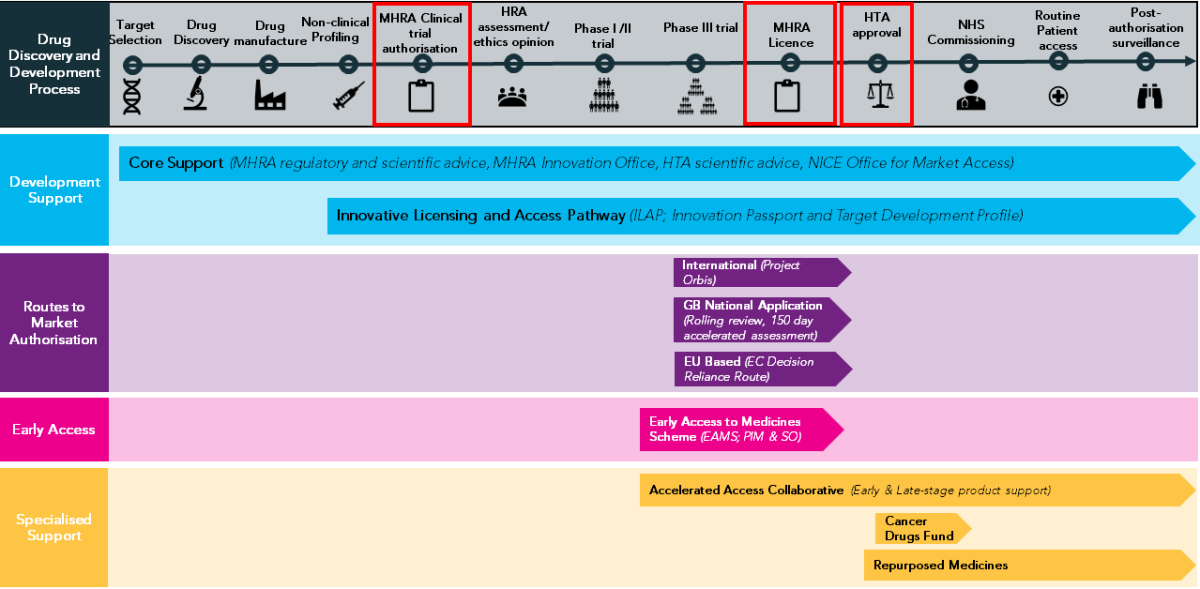
Key information from the paper that can also be viewed on the ECMC Website:
