Barts ECMC launch patient videos in multiple languages to engage diverse communities in clinical trials
16 Jan 2024
Barts ECMC and Barts Health NHS trust covers the largest population in the UK with 2.6 million people across North and East London. The Barts ECMC team recruits a high number of patients onto many clinical trials each year. The team identified that the patients enrolling onto clinical trials were not fully representative of the diverse population of their local community in East London. Through conversations with patients and families, the team identified that this is in part due to language barriers and cultural beliefs that may limit accessibility to trials. The team embarked on a project to increase access to trials by improving information materials for potential patients and their families. The project involved creating patient-facing videos where patients were interviewed in their native language about their experience of being on a clinical trial to provide more clarity about the process, deliver an honest account of their experiences, and to bust some potential misconceptions about clinical trials in general. Find out more about this project.
The team hope these videos will make a difference in increasing engagement and accessibility to clinical trials. In this interview we hear from one of the patients involved in the process, Beata Plywacz, as well as CRUK Senior Research Nurse Maria Lapuente and Dr Francesca Jackson-Spence from Barts ECMC.
How did you come to recognise this was a challenge that needed addressing?
Barts ECMC Team: We analysed demographic data of lung cancer trial patients versus standard of care patients, and it was clear that some communities were not appropriately represented in trials, compared to the demographic of the population our ECMC covers.
The team noted that those from communities with English not as a first language and those from deprived communities were particularly under-represented.
We also know from our existing patients that enrolling onto a clinical trial can be a daunting process for patients. Patients told us the reassurance they got from clinical staff about what was to come and having the opportunity to express their concerns and have their questions answered helped relieve some anxiety. Unfortunately, due to language barriers, those patients who don’t speak English or don’t have English as a first language don’t get the same level of rapport with the trials team. It’s important to us that all patients make informed choices and have the same opportunity to access clinical trials as anyone else.
We have conducted a service evaluation of recruitment in a sub-section of clinical trials1 which provided a clearer picture of disparities of some background when it comes to trial participation:
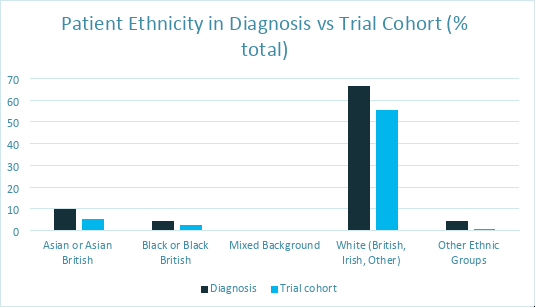
1. Service evaluation of diversity recruitment in thoracic cancer clinical trials at a tertiary cancer centre: reducing barriers to recruitment (F. Chamberlain, M. Lapuente, P. Kirby, A. Adebamowo, K. Lau, W. Ricketts, J. Conibear, J. Steele, P. Szlosarek, F. Lim and A. Januszewski (2022) (Lung Cancer vol.165). Data collected from 1st January to 31st December 2019.
These videos have been translated into a number of languages. How did you identify which languages would be most beneficial to the local communities?
Barts ECMC Team: We conducted an internal data collection to understand the percentage of different communities within the population Barts ECMC serves. This showed that our demographic includes high numbers of those speaking non-English languages, in particular those speaking Bengali, Turkish, Polish, and Hindi. However, these numbers didn’t translate into recruitment onto clinical trials.
Why did you decide on videos as a good resource to begin to address this issue?
Barts ECMC Team: The majority of clinical trial information provided is written. Not only can it be overwhelming to add further written documents for potential patients/families to read, but it also further excludes those with lower literacy abilities or those who don’t have English as a first language. Video content allows us to present the information in the patient’s own language, tone and allows their genuine experience to be shown.
We asked patients and members of our PPIE group how best they would like to receive this information, and video content with visual representation was the most popular.
Beata Plywacz: In my opinion, the video format is very effective when you can see a face of trial’s representative or patient. It looks more real and friendly as opposed to just written documentation. It also feels more trustworthy and engaging. In addition, in my experience with people that I know and have spoken with about their experiences, people whose first language is not English would understand more from an informal conversation than from any written information.
What are the key messages you are trying to get across in these videos?
Barts ECMC Team: The main goal was challenge myths or cultural beliefs about clinical trials that may be potential barriers to trials. Unfortunately, despite the positive benefits of clinical trials, our research shows that some people still believe they are ‘guinea pigs’ or are on a trial to help future patients, with no benefit to their own care. We wanted to showcase patients’ own genuine and honest experience of trials to help desti
gmatise clinical trials and break down some of the barriers to patients accessing the same opportunities.
The aim of the videos is to move away from the idea of research being done to patients to an idea of research being done with patients. We wanted to truly recognise that patients were more than just research participants, but had feelings and are the most important element of the trial, with patients being seen to be at the top of the research pyramid, and to emphasise that clinical trials are available to everyone, regardless of race, cultural, background or socioeconomic status.
We also wanted to be realistic and transparent about the pros and cons of being involved in research, helping potential patients make an informed choice. We are grateful to our patients featured in the video for being so honest about not only the positives of their trial, but also the more challenging aspects of being involved and for highlighting that what one person may see as a benefit (e.g. more engagement with the site and staff/more hospital visits) may not be everyone’s preference.
Beata Plywacz: It was all about emphasising that involvement in trial was all about giving yourself more chances and ultimately bringing hope to patients, with the awareness that new treatments are constantly being developed and a new breakthrough could be right around the corner. It was really important to ensure potential participants in trials were aware of what research was and what new treatment options were in the pipeline to boost their confidence.
It was also important to me to highlight that you did feel part of a team being involved in the trial and that having that additional support and care was really helpful.
How did you engage with patients to develop these videos?
Barts ECMC team: We wanted to include a range of patients from different cancer types, age groups and backgrounds. We reached out to each of the cancer trials groups to gauge involvement interest amongst their patients. We then reached out to these patients and discussed our intention and welcomed their feedback and impact.
It was really important to use to develop the recorded questions in collaboration with the patients and did so with the ECMC Patient and Public Involvement and Engagement (PPIE) group.
We then invited the patients in to the hospital to be interviewed by us, and the hospital communications team provided a videographer. Recording the videos was a really exciting experience for both us and the patient and allowed us to get to know our patients better.
Beata, why did you think it was important to be involved in this activity?
Beata Plywacz: It’s very important for more diverse groups to understand what a clinical trial is and what it means to be on a trial. It was especially important to ensure that people who don’t speak English weren’t missing out on opportunities to learn about trials. These videos allowed information to be provided in a more understandable way and hopefully seeing someone from your community going through this experience will make others more likely to want to be involved.
Beata, how did you find the experience working with Barts ECMC?
Beata Plywacz: It was a very positive experience, and it was great to work with the trial teams who were very clear and honest. I’ve built strong relationships with the team and am happy to have been involved in such a project to improve the understanding of trials in communities such as mine.
One challenge I faced was actually speaking about some of these terms in Polish. Despite it being my first language, it was sometimes difficult to identify the current Polish terms for trial-specific phrases that I was only familiar with in English. However, working with the ECMC team and taking a considered approach to the content of the videos meant that we achieved clear and concise information for the Polish-speaking community.
I’m pleased to have been involved in the development of the videos and am confident they will be a really helpful resource for those looking to be involved in clinical trials in the future.
If you were to do this again, what would you do differently? Are there any next steps planned?
Barts ECMC Team: We would like to be reaching wider populations and would have liked to include more languages/more translation into other languages in these videos.
More widely, we have implemented multiple new measures to ensure equity, diversity, and inclusion in clinical trials. We are working with other centres across the Network and directly with the private sector to develop effective strategies for engagement, involvement, and recruitment.
We are also already collecting internal data across all tumour types to analyse the EDI in clinical trials from the implementation of all these measures until June 2023. This will give us more information to identify what areas we should be focusing on in the future to address the needs of all our patients.
We will also be collaborating closely with the hospital communications team to get the videos distributed further.
Any other final thoughts
Barts ECMC Team: Whilst we recognise we still have a long way to go improving access to clinical trials, we hope these videos can be shared amongst other patients/centres and provide some relatable, honest and insightful perspectives on the patient experience of clinical trials to potential patients. Enrolling on a clinical trial can be a frightening experience and we hope seeing the patients’ smiley faces and true accounts of their journey provide some reassurance during this challenging time.
