The industry engagement work of the ECMC Programme Office
19 Jul 2022
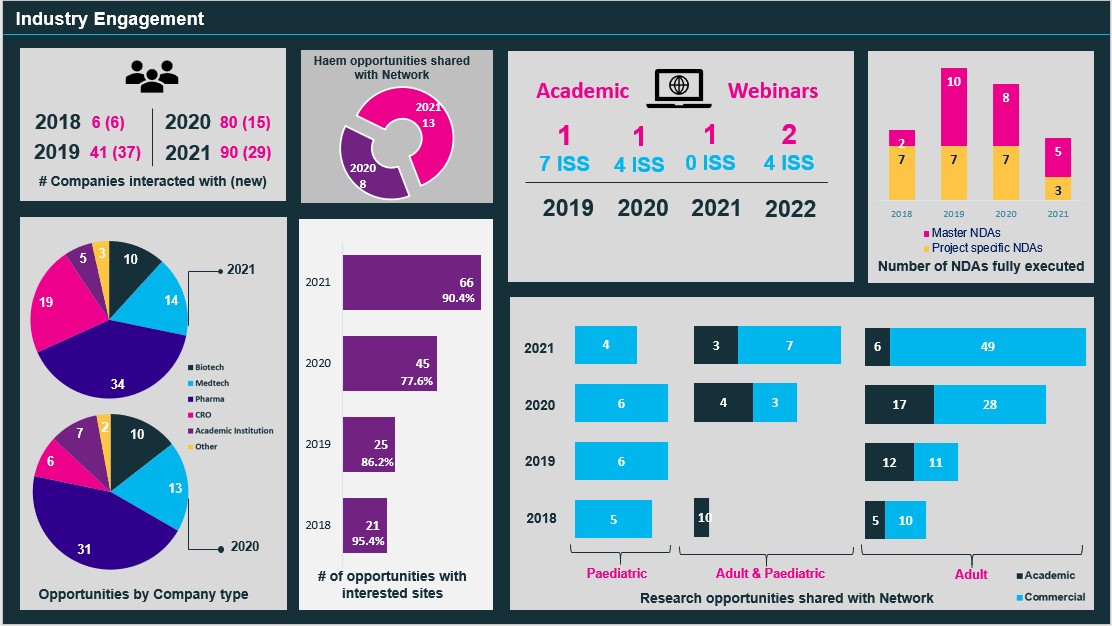
The ECMC Programme Office, since 2018 has focused on promoting the network externally (via national and international conferences) and building relationships not just with Pharma, Biotech, Medtech and CROs but with key stakeholders including our sites, regulators and the various teams within the NIHR infrastructure who engage with industry. Our work has seen an increase in trial opportunities submitted to the Network and the number of companies interacted with (see infographic above).
To date our approach has resulted in a significant increase in commercial trial opportunities (15 in 2018; 70 in 2021) coming to the network via the Programme Office. Site responsiveness to commercial trial opportunities is high with 90.4% of opportunities submitted to the Network resulting in at least 1 site expressing interest in participating in 2021. This suggests the quality of trial information shared is appropriate (majority under a network NDA) and leads to repeat requests for trial placement support from Industry, further supported by our move to an online submission process accessed via our website.
To further grow our relationships, the Programme Office developed and implemented an industry/academic collaboration framework. The framework supports pharma to access academic insight to inform clinical development plans or trial protocol advice, in addition to supporting engagement to progress Investigator Trial Proposals. To date the Programme Office have hosted 6 webinars with 5 companies leading to 15 trial proposals with the first webinar held in 2019. These webinars support the discussion of both pre-clinical and clinical data under a network NDA and enable rich discussion on future development plans.
The Programme Office regularly meets with Sponsors to raise awareness of the Network, understand challenges and position the UK as the place of choice for early phase oncology research. Members of the Programme Office team attended ASCO in June 2022 (after a 2-year hiatus) and met with several companies to discuss collaboration opportunities for both the Adult and Paediatric Networks, following engagement via the ECMC Industry Newsletter which is published three times a year to coincide with significant Oncology Conferences (AACR/ASCO, ESMO and ASH).
With respect to our paediatric and TYA activities, since 2018, more than 30 opportunities have been shared with the Paediatric Network and we have recently had 3 additional companies express interest in signing our master paediatric NDA bringing our total to 3. We also plan, for the first time, to meet with Pharma partners at the upcoming SIOP International conference in Barcelona and discuss opportunities for the ECMC to support company’s early phase CYP development plans.
Looking ahead, whilst we continue to support large pharma, we are increasingly engaging with smaller biotech from the UK, US and Europe to support their transition to FIH research. We are seeing an increase in Medtech opportunities coming to the network and continue to build our engagement with CROs, utilising the Network Intelligence dashboards to inform our view of the networks capabilities.
